sp hybridization.
The process of mixing one s- orbital with one p- orbital in an atom to form two sp hybrid orbitals of equivalent energy is called sp hybridization.
EXAMPLE – ACETYⅬENE / ETHYNE
In the acetylene molecule, the central carbon atom is sp hybridized.
Carbon (6) 1s2 2s2 2p2→ Ground state.
C(6) 1s2 2s1 2p3 → One 2s electron is promoted to 2p orbital.
Then, 2s and one 2p orbital hybridize to form two sp2 hybrid orbitals.
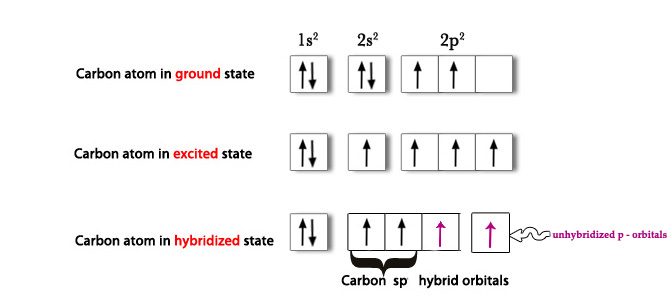
As seen in the above figure, there are two unpaired electrons in two unhybridized orbitals. As they are unpaired, they have to react in some way to pair themselves up and thus become more stable. These unhybridized orbitals, overlap laterally to form two π bonds. Thus, acetylene has a triple bond – 1 sigma bond (sp-sp overlap) and two π bonds (p-p overlap).
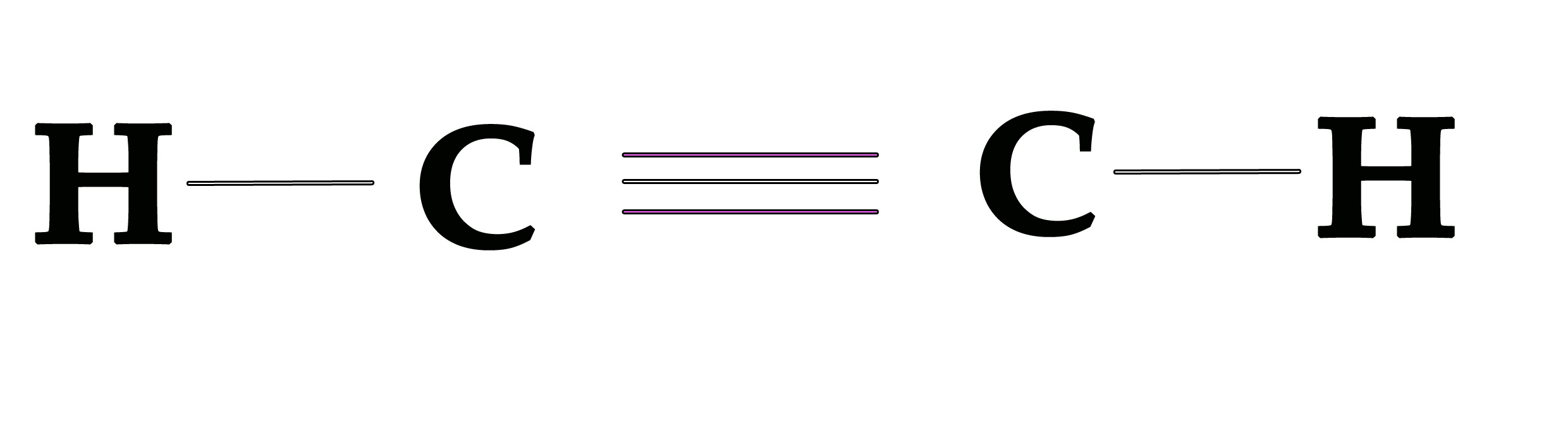
Pictorially the molecule can be represented as shown below –
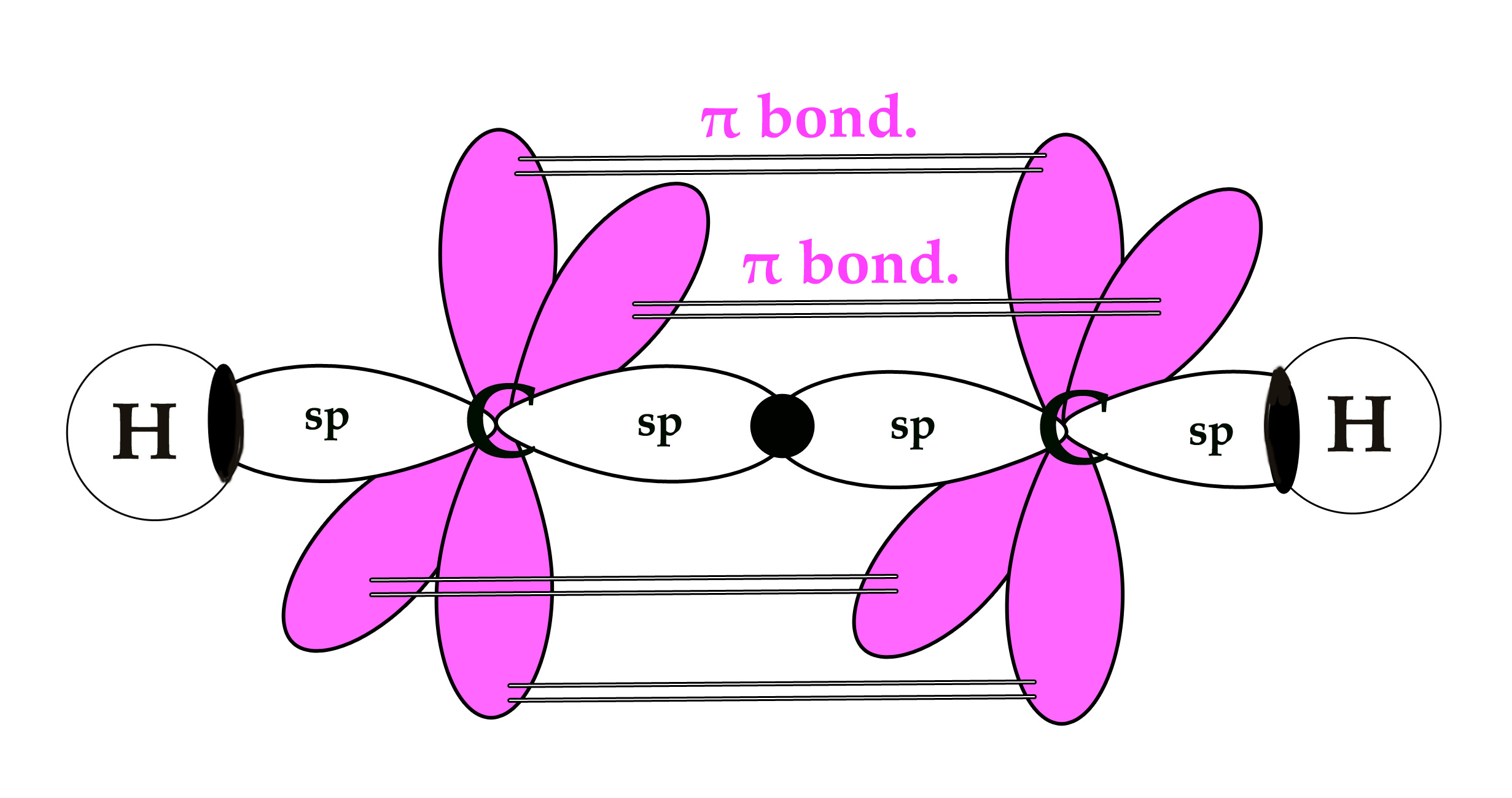
The bond angle is 180º and the molecule has linear geometry. Other molecules with sp hybridized central atom are BeF2,BeCl2 ,XeF2.
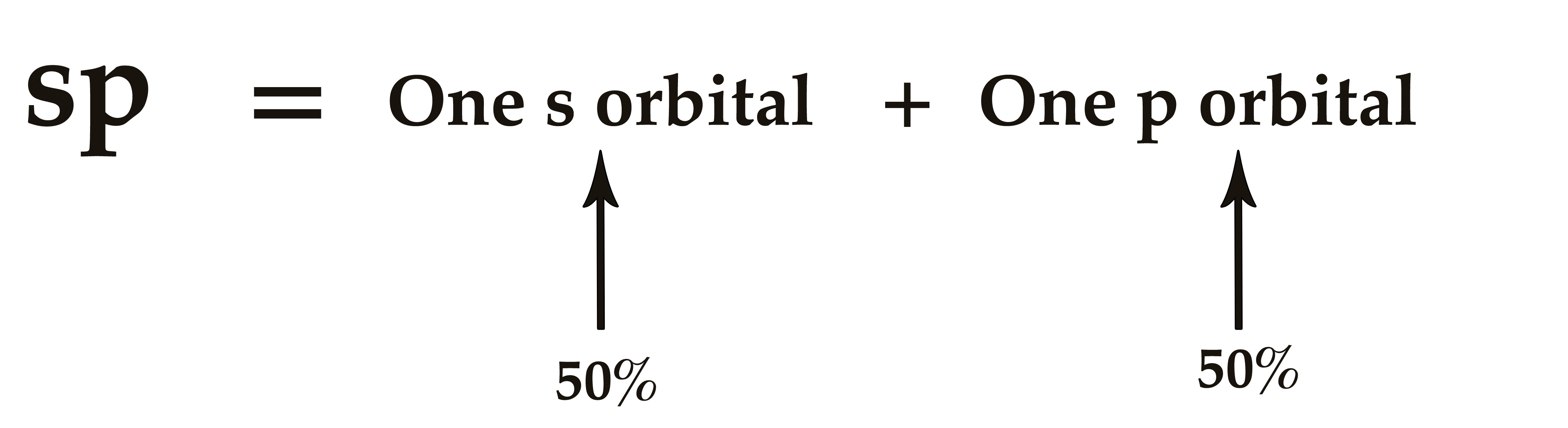
An sp hybrid orbital has 50% s-character and 50% p-character.
sp hybridization occurs when a carbon atom is attached to 2 groups. In the above example, each carbon atom is attached to one hydrogen atom and another carbon atom.
The following videos will help us visualize the hybridization concept better –
With this post, we have looked into the basic types of hybridizations. In the next post, we shall see some more types of hybridizations. Till then,
Be a perpetual student of life and keep learning…
Good Day!